- If you have any questions, please contact us!
- +84 965 624 065
- info@greennrj.com.vn

Complete Guide to Letter of Authorization (LOA) for Cosmetic Product Notification in Vietnam 2025 | Avoid Costly Import Mistakes
April 15, 2025
How to Set Up Construction Company in Vietnam (2025) – Successful Guide for Foreign Investors
April 16, 2025Want to Import Cosmetic Product Formula into Vietnam in 2025? One crucial step is preparing a fully compliant Cosmetic Product Formula. This formula plays a key role in your cosmetic notification dossier, helping ensure your products meet all Vietnamese cosmetic regulations as well as the ASEAN Cosmetic Directive (ACD) standards. In this 2025 guide, Green NRJ provides a comprehensive overview of the legal requirements, import procedures, and registration process for cosmetic products in Vietnam, helping you successfully navigate the regulatory landscape.
What is a Cosmetic Product Formula?
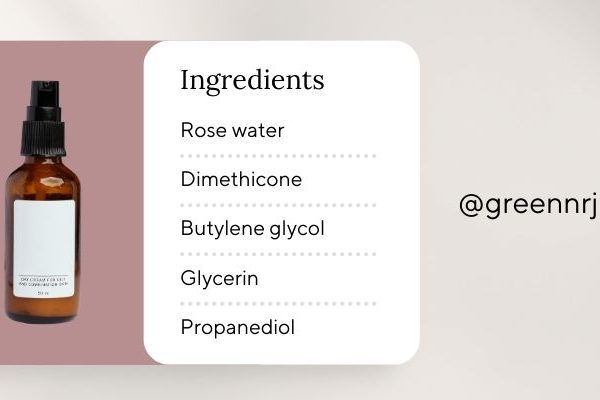
A Cosmetic Product Formula is a detailed list of all ingredients used in a cosmetic product. It is a mandatory part of the cosmetic notification dossier required to legally import cosmetics into Vietnam. This formula ensures transparency, product safety, and compliance with both Vietnamese cosmetic regulations and the ASEAN Cosmetic Directive (ACD).
Legal Basis for Cosmetic Formula Submission in Vietnam
To successfully register and import a cosmetic product into Vietnam in 2025, your product formula must comply with the following legal frameworks:
-
ASEAN Cosmetic Directive (ACD): Sets ingredient safety standards and permissible usage for all cosmetic products across ASEAN countries, including Vietnam.
-
Circular No. 06/2011/TT-BYT: The core Vietnamese regulation governing cosmetic product management, including formula requirements.
-
Circular No. 32/2019/TT-BYT: Updates and supplements Circular 06, with specific guidance on cosmetic documentation and ingredient declarations.
Submitting a compliant cosmetic product formula is not optional—it is a legal requirement to import, distribute, and sell cosmetics in Vietnam.
Mandatory Information in the Cosmetic Product Formula
When importing cosmetics into Vietnam in 2025, your Cosmetic Product Formula must be meticulously prepared to comply with Vietnamese regulations and the ASEAN Cosmetic Directive (ACD). Below are the essential elements that must be included in the formula as part of the cosmetic notification dossier:
1. INCI Ingredient Listing (International Nomenclature of Cosmetic Ingredients)
-
All cosmetic ingredients must be listed using their official INCI names, as required by both the ASEAN Cosmetic Guidelines and Vietnam’s Ministry of Health.
-
Ingredients must be arranged in descending order by concentration—from highest to lowest percentage.
-
Ingredients used at concentrations below 1% may be listed after those used at 1% or higher, in any order.
-
Fragrances and flavoring agents can be declared collectively as “Fragrance” or “Perfume”, following international labeling standards.
-
Colorants must be identified using the CI number (Color Index) or as per Annex IV of the ASEAN Cosmetic Directive, which lists permitted colorants.
2. Ingredient Percentages and Regulatory Compliance
-
Each ingredient in the cosmetic formula must clearly state its exact concentration (e.g., 0.5%, 1.2%), which is crucial for regulatory review.
-
All ingredient concentrations must comply with the limits set in the ASEAN Annexes (especially Annex III and VI for restricted substances and preservatives).
-
The formula must ensure the product meets safety standards for heavy metal content (e.g., lead, arsenic) and microbiological contamination limits, in accordance with ASEAN technical requirements.
-
Only substances approved for use in cosmetics under the ASEAN Cosmetic Guidelines are allowed—prohibited or restricted ingredients must be avoided or used within permitted limits.
3. Use of Scientific Names for Natural-Derived Ingredients
-
Botanical ingredients must be listed with their full scientific (Latin) names, including genus and species (e.g., Camellia sinensis extract for green tea).
-
Animal-derived ingredients, if used, also require scientific classification, and must adhere to import restrictions or additional documentation under Vietnamese law.
-
This scientific accuracy helps regulatory authorities verify ingredient safety, origin, and compliance with both ASEAN and Vietnamese cosmetic standards.
Ingredients Not Considered Part of the Formula
When preparing your cosmetic product formula for importation into Vietnam, it’s important to understand which components are excluded from the formula declaration. According to the ASEAN Cosmetic Directive (ACD) and Vietnamese cosmetic regulations, the following are not considered part of the official formula:
-
Trace impurities present in raw materials that have no impact on product safety and are unintentionally present (e.g., residual solvents or process-related by-products).
-
Processing aids or technical materials that are used during manufacturing but are not present in the final product, such as filtration agents or catalysts.
-
Volatile solvents or carriers used in fragrances or flavorings that completely evaporate during production, leaving no trace in the finished cosmetic product.
These exclusions help regulatory authorities focus on ingredients that actually remain in the product and impact consumer safety.
Common Mistakes to Avoid
Failure to comply with current Vietnamese cosmetic regulations or the ASEAN Cosmetic Directive (2025 version) can lead to rejection of your cosmetic notification dossier, delays in customs clearance, or even import bans. Below are the most frequent errors cosmetic importers make when preparing the product formula:
-
Using Trade Names Instead of INCI Names
-
All ingredients must be listed using the International Nomenclature of Cosmetic Ingredients (INCI). Brand names, supplier-specific codes, or proprietary labels are not accepted.
-
-
Including Banned or Restricted Ingredients
-
Ingredients listed in Annex II of the ACD are strictly prohibited in all ASEAN countries, including Vietnam. Using them—even unintentionally—will result in automatic rejection.
-
If using ingredients listed in Annex III, IV, VI, or VII, you must ensure that concentration limits and usage conditions are fully met.
-
-
Failing to Ensure Total Formula Adds Up to 100%
-
The complete ingredient list must account for exactly 100% of the product composition. Any deviation suggests inaccuracies in the formulation and can raise red flags during dossier evaluation.
-
-
Submitting Outdated or Incomplete Formulas
-
Cosmetic regulations are updated regularly. Submitting a formula that does not reflect the latest 2025 regulatory updates (including ingredient safety, maximum concentrations, or banned substances) may render your application invalid.
-
-
Lack of Scientific Names for Natural Ingredients
-
Failing to include Latin names for botanical extracts or animal-derived substances can delay approvals, as regulators require full traceability and identification for natural ingredients.
-
Frequently Asked Questions (FAQs) about Import Cosmetic Product Formula into Vietnam
What is a Cosmetic Product Formula in the context of importing to Vietnam?
A Cosmetic Product Formula is the detailed composition of ingredients used in a cosmetic product. It must comply with Vietnamese regulations and ASEAN Cosmetic Directive standards to be approved for import and sale.
Is the Cosmetic Product Formula required for all imported cosmetics in Vietnam?
Yes, submitting a compliant Cosmetic Product Formula is a mandatory part of the cosmetic notification dossier for all imported cosmetics entering Vietnam.
What are the main legal documents governing the import of cosmetic formulas into Vietnam?
The import process is governed by laws such as Circular 06/2011/TT-BYT, Decree 69/2018/NĐ-CP, and relevant ASEAN cosmetic regulations.
How long does the registration and approval process typically take?
The process usually takes between 30 to 60 working days, depending on the completeness of the dossier and regulatory body workload.
Can foreign manufacturers submit the Cosmetic Product Formula directly?
Typically, a local authorized representative or importer submits the dossier on behalf of the foreign manufacturer.
Green NRJ’s Cosmetic Compliance Services
At Green NRJ, we support local and foreign companies to import cosmetics into Vietnam with ease.
Our services include:
- Reviewing your Cosmetic Product Formula for ASEAN compliance
- Ingredient validation and safety checks
- Preparation of cosmetic notification dossiers
- Acting as your authorized representative in Vietnam
Need help with your cosmetic product formula or full notification process? Contact Green NRJ now for expert guidance.
Related Articles
- How to Import and Sell Cosmetics in Vietnam
- Comprehensive Guide to Cosmetic Registration in Vietnam
- Comparison: Company vs. Cosmetic Notification Holder in Vietnam
- How to Group Multiple Cosmetic Products Under One Notification
- PIF for Cosmetics in Vietnam: What It Is and Why It Matters
- Everything About Cosmetic Advertising in Vietnam (2025 Guide)
- Cosmetic Notification Holder – Import Without Opening a Company