- If you have any questions, please contact us!
- +84 965 624 065
- info@greennrj.com.vn

Importer of Record (IOR) Services in Vietnam
March 15, 2025
Guide to Importing Infant Formula Milk into Vietnam
March 17, 2025Medical device registration in Vietnam is essential for any manufacturer or distributor looking to tap into Vietnam’s fast-growing healthcare market in 2025. With rising demand, stricter regulations, and increasing investments in healthcare infrastructure, ensuring proper registration not only secures legal market access but also builds trust with local hospitals and patients. This comprehensive guide explains the entire registration process, from device classification and dossier preparation to labeling, import licensing, and post-market obligations, helping you navigate Vietnam’s evolving regulatory landscape confidently and efficiently.
Legal Framework for Medical Device Registration in Vietnam
Legal Foundation
-
Decree No. 98/2021/ND-CP (effective 2022): Governs management, registration, import, distribution, and use of medical devices.
-
Decree No. 07/2023/ND-CP: Amends and supplements several provisions of Decree No. 98/2021/ND-CP, updating procedures and compliance requirements for medical devices, including conditions for circulation, registration number issuance, standard declaration, and price listing of medical devices.
-
Circular 05/2022/TT-BYT: Details device classification and dossier requirements.
-
Circular 18/2022/TT-BKHCN: Labeling and packaging compliance.
Competent authorities in charge
The MOH, via its specialized body—DMEC—is the key authority overseeing medical device registration process Vietnam, product evaluation, approval, and post-market surveillance. DMEC ensures all medical devices meet Vietnam’s safety, quality, and performance standards before they are permitted to circulate in the Vietnamese market.
Vietnam Medical Device Classification: Risk Classes and Importance
The Vietnam medical device classification system is a fundamental part of the medical device registration in Vietnam process. In line with Circular 05/2022/TT-BYT and the ASEAN Medical Device Directive (AMDD), devices are categorized from Class A to Class D, based on intended use and risk level. Accurate classification directly affects regulatory pathways, required documents, and approval timelines.
Devices are classified into four risk-based categories:
-
Class A (Low Risk)
General medical devices with minimal risk to users or patients.
Examples: Medical bandages, surgical gloves, cotton swabs, stethoscopes. -
Class B (Low to Moderate Risk)
Devices that present slightly more risk than Class A and may require more oversight.
Examples: Hypodermic syringes, diagnostic ultrasound equipment, infusion sets. -
Class C (Moderate to High Risk)
Devices that support or sustain life, pose moderate risk, or are used in critical situations.
Examples: Infusion pumps, anesthesia machines, blood dialysis equipment, ventilators. -
Class D (High Risk)
Implantable or life-sustaining devices with the highest level of risk.
Examples: Pacemakers, implantable defibrillators, heart valves, orthopedic implants.
Important Classification Guidelines:
Under the latest regulations, the registration holder (importer or authorized representative) is now responsible for self-classifying their devices and fully accountable for the accuracy of this classification. This means companies must carefully assess and declare the correct risk class in their dossiers and are legally responsible for any errors.
For combination or multifunctional devices, classification must follow the component with the highest risk. To minimize risks of delays, penalties, or market suspension, it is strongly advised to consult local regulatory experts or legal advisors to ensure proper compliance with Vietnam’s updated framework.
Essential Procedures and Compliance for Medical Device Registration in Vietnam
Vietnam enforces clear and detailed medical device registration requirements, outlining procedural steps and documentation obligations that all manufacturers and distributors must follow to obtain timely approval.
For Class A and B devices, the process mainly involves submitting a Declaration of Applicable Standards through the DMEC online portal, making it faster and less complex.
Meanwhile, Class C and D devices require full registration if listed in the licensing-required category under Circular 05/2022/TT-BYT. However, this circular also specifies certain exemptions where devices do not need marketing authorization but still must be properly classified and comply with all technical standards before being imported or distributed.
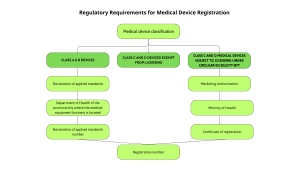
Class A and Class B Medical Devices (Low to Moderate Risk)
Registration Method:
For Class A (low risk) and Class B (low to moderate risk) medical devices, manufacturers or importers submit a Declaration of Applicable Standards via the DMEC online portal. The registration is then managed by the Provincial or City Department of Health where the business is based.
This streamlined procedure enables faster market access by focusing on declared standards rather than requiring a full technical evaluation. Under current regulations, the registration holder is responsible for self-classifying the device and fully accountable for the accuracy of this classification.
Approval Timeframe:
Typically, registration approval is immediate or granted within a few business days after submission, assuming all required documents are complete and accurate.
Essential Documentation Includes:
| Document | Description |
|---|---|
| Completed Declaration Form | Prepared according to Circular 05/2022/TT-BYT. |
| Classification Result | Confirms the device is classified as Class A (low risk). |
| Product Documentation | User manuals and instructions for use, translated into Vietnamese to ensure safe operation. |
| Certificate of Free Sale (CFS) | Issued by the competent authority in the manufacturing country, confirming legal marketing and distribution. |
| Quality Management System Certificate | Proof of compliance with a recognized QMS, such as ISO 13485. |
| Letter of Authorization or Power of Attorney | Issued by the manufacturer or brand owner, appointing the importer or local representative in Vietnam. |
| Warranty Policy Document | States the product’s warranty terms or confirms that no warranty obligations apply. |
Class C and Class D Medical Devices (Moderate to High and High Risk)
Registration Method:
For Class C and D devices, registration depends on inclusion in the list of devices requiring marketing authorization Vietnam (MA) as detailed in Circular 05/2022/TT-BYT. Devices on this list must submit a full registration dossier to DMEC and receive an MA certificate before legal marketing. Devices exempt from this list do not require MA but must still undergo classification and meet applicable standards.
Devices requiring a Marketing Authorization
If the medical device is included in the list of devices that require a license, the following procedure must be followed:
-
Submit a complete Marketing Authorization application to the Department of Medical Equipment and Construction (DMEC), under the Ministry of Health (MOH).
-
The application is reviewed by the MOH’s Appraisal Council.
-
Upon approval, the applicant will receive a Certificate of Registration along with a Registration Number.
-
Only after obtaining this Certificate and Registration Number can the Class C or D medical device be legally circulated in Vietnam.
This process is mandatory for all moderate to high-risk medical devices that fall under the controlled list in Vietnam.
Devices exempt from Marketing Authorization
If the Class C or D medical device is not listed in the licensing-required category (i.e., it is included in the exemption list under Circular 05/2022/TT-BYT), then:
-
No Marketing Authorization or Certificate of Registration is required.
-
Instead, the business only needs to conduct the Medical Device Classification process to determine the appropriate risk class.
-
Once the classification is approved, the device can be imported, distributed, and used without undergoing full registration.
This exemption significantly reduces the time and administrative burden for businesses dealing with low-volume or lower-risk Class C and D devices.
Approval Timeframe for Marketing Authorization
The typical timeframe for evaluation and issuance of a Marketing Authorization (MA) and Certificate of Registration for Class C and D devices is 30 to 60 working days from the date of formal application acceptance by DMEC. Processing duration may vary based on dossier completeness, DMEC workload, and device complexity.
However, this timeframe for registration approval may vary depending on several factors, including:
-
The completeness and accuracy of the submitted dossier
-
The current workload and processing capacity of the DMEC
-
The need for additional documents or clarifications during the appraisal process
-
The complexity or novelty of the medical device
Applicants are advised to ensure all documents meet the required standards to avoid delays and to monitor the process closely via the DMEC online portal.
Comprehensive Documentation Checklist for Marketing Authorization
The registration dossier must include detailed technical documentation as required by Circular 05/2022/TT-BYT, including quality control reports, design documentation, maintenance instructions, and related certificates.
| Document | Details & Requirements |
|---|---|
| Written Request for New Circulation Number | Official application letter prepared by the registrant, requesting issuance of a Marketing Authorization Number. |
| Medical Device Classification Result | Classification report confirming the device’s risk class (C or D), in accordance with Decree 98/2021/NĐ-CP and Circular 05/2022/TT-BYT. |
| ISO 13485 Certificate | Scanned copy proving compliance with international quality management standards. |
| Certificate of Free Sale (CFS) | Must be legalized and translated into Vietnamese; proves the product is legally marketed in other countries. |
| Letter of Authorization / Power of Attorney | Issued by the manufacturer or brand owner; must be notarized, legalized, and translated into Vietnamese. |
| Warranty Confirmation Letter | Legalized and translated confirmation of warranty terms or declaration that the product is not subject to warranty. |
| Clinical Evidence or SSCP | Scanned copy providing safety and clinical performance data (e.g., clinical evaluation report or Summary of Safety and Clinical Performance). |
| Product Catalog | Scanned copy detailing product specifications, features, and available variants. |
| User Manual | Scanned copy, typically in English and/or Vietnamese, guiding safe and correct use. |
| Product Labels | Scanned copies of main and secondary labels, ensuring full compliance with local labeling regulations. |
| Business Registration Certificate | Certificate of the authorized Vietnamese representative or importer, proving legal entity status. |
| CSDT Inspection Record & CSDT Dossier | Required after submission; includes Common Submission Dossier Template (CSDT) and inspection record. |
| Account & Password for Online Portal | Required to access and submit applications via Vietnam’s medical device registration system. |
Additional Considerations:
-
For Class D devices, particularly those that are implantable or life-sustaining (e.g., pacemakers, heart valves), clinical trial data may be required if the device lacks prior regulatory approval or significant use in developed countries. This ensures the highest patient safety standards and regulatory compliance.
5. Step-by-Step Medical Device Registration Process
Successfully registering medical devices in Vietnam requires following a clear, regulated process designed to ensure product safety and regulatory compliance. Below is a detailed step-by-step procedure for foreign manufacturers and distributors aiming to enter the Vietnamese medical device market in 2025.
Step 1: Appointment of a Local Authorized Representative (Legal Entity)
-
For Class C and D devices, foreign manufacturers must appoint a Vietnam-based Marketing Authorization Holder (MAH) — a local legal entity or authorized agent — to manage regulatory submissions, hold the Marketing Authorization (MA) number, and ensure ongoing compliance.
-
For Class A and B devices, manufacturers only need a local authorized representative (registration holder) to handle the declaration, submit documents, and maintain regulatory communications.
Step 2: Self-Classification
-
Before registration, the registrant holder/ Marking Authorization Holder must confirm and finalize the device’s classification result, which serves as the foundation for preparing the required dossier and choosing the correct regulatory pathway.
Step 3: Dossier Preparation According to Device Class
-
Prepare a comprehensive registration dossier tailored to the device class (A, B, C, or D) as per Vietnamese regulations.
-
Notarize or legalize key documents as required to meet Vietnam’s regulatory standards.
Step 4: Application Submission via DMEC Online Portal
-
For Class A and B devices:
Submit a Declaration of Applicable Standards through the official DMEC online portal. This streamlined process facilitates faster approval for low and moderate-risk devices. -
For Class C and D devices:
Submit the full registration dossier electronically via the DMEC portal. The Ministry of Health will conduct a detailed technical evaluation, which may include requests for supplementary information or clarifications.
Step 5: Evaluation, Approval, and Issuance of Marketing Authorization Number
-
Class A and B devices:
Once the application is complete and compliant, the system usually grants instant or near-instant approval, generating the registration number immediately. -
Class C and D devices:
The MOH will perform a comprehensive dossier review, taking approximately 30 to 60 working days. Upon successful evaluation, the Ministry will issue the Marketing Authorization (MA) number, allowing legal importation and distribution within Vietnam.
Following this medical device registration process in Vietnam ensures compliance with the Vietnam Ministry of Health medical device regulations, timely approval, and legal market access.
Why Following This Registration Procedure is Vital for Market Access in Vietnam
Adhering strictly to this step-by-step registration process ensures:
-
Compliance with Vietnam’s updated 2025 medical device regulations
-
Faster approval timelines by minimizing procedural errors
-
Avoidance of costly delays or application rejections
-
Legal market access and enhanced credibility with healthcare providers and regulators
For foreign medical device companies seeking growth in Vietnam’s rapidly expanding healthcare market, understanding and executing this registration workflow with precision is essential for sustainable business success.
6. Role of the Marketing Authorization Holder (MAH)
Foreign medical device manufacturers must appoint a local entity in Vietnam to act as the Marketing Authorization Holder (MAH). The MAH is the official legal representative responsible for all regulatory matters related to the device registration and market compliance.
The MAH is a legal entity registered in Vietnam and bears full responsibility for the registered medical device. This includes compliance with relevant laws and regulations, monitoring product quality after market entry, reporting adverse events, and cooperating with regulatory authorities in case of product recalls.
Key Responsibilities of the MAH:
-
Regulatory Submissions: Submit and manage registration dossiers, renewals, and updates with the Ministry of Health (MOH) and Department of Medical Equipment and Construction (DMEC).
-
Legal Compliance: Ensure all registered devices comply with Vietnamese laws, standards, and safety requirements throughout their lifecycle.
-
Post-Market Surveillance: Monitor device performance after market entry, report adverse events, and handle recalls if necessary.
-
Communication: Act as the main liaison between foreign manufacturers, Vietnamese regulators, distributors, and healthcare providers.
-
Label and Document Management: Ensure Vietnamese labels, instructions, and promotional materials meet local regulatory standards.
Appointing a reliable MAH is crucial for foreign companies to ensure smooth registration, maintain compliance, and access the growing Vietnamese medical device market effectively.
7. Vietnam Medical Devices Labeling Regulation
Mandatory Label Information for Medical Devices
According to Vietnamese regulations including Decree 43/2017/ND-CP, Decree 111/2021/ND-CP, and Circular 18/2022/TT-BKHCN, all medical device labels distributed in Vietnam must clearly display the following information in Vietnamese language:
| Label Element | Description |
|---|---|
| Medical Device Name | Official product name registered with the Ministry of Health (MOH). |
| Manufacturer/Responsible Party | Full name and physical address of the manufacturer, importer, or responsible entity. |
| Country of Origin | Country where the device was manufactured or the final processing location. |
| Registration or Import License Number | Valid Marketing Authorization (MA) or import license number issued by MOH. |
| Batch Number or Serial Number | Unique identifier for batch or device serial number to enable traceability and recall. |
| Manufacture Date and Expiry Date |
|
| Instructions for Use and Storage | Clear usage instructions and recommended storage conditions to ensure safety and product quality. |
| Warnings and Precautions | Safety warnings, contraindications, or precautionary notes if applicable. |
| Language Requirement | All labeling must be in Vietnamese. Foreign languages must be accompanied by a full Vietnamese translation. |
Consequences of Non-Compliance with Labeling Regulations
According to Decree 43/2017/ND-CP and subsequent amendments such as Decree 111/2021/ND-CP, medical device labeling must fully comply with mandatory Vietnamese-language information requirements. This includes the device name, manufacturer location, registration number, manufacturing and expiry dates, instructions for use, and safety warnings. Additionally, all accompanying promotional materials must adhere strictly to content and language regulations.
Failure to meet Vietnam’s stringent labeling requirements can lead to:
-
Regulatory penalties and fines
-
Delays or suspension of device registration approval
-
Forced product recalls or market withdrawals
-
Damage to manufacturer reputation and loss of market access
Why Proper Labeling is Critical for Medical Device Success in Vietnam
Compliant labeling not only ensures legal market access but also builds user trust and supports effective post-market surveillance and safety monitoring. Manufacturers and distributors should prioritize thorough review and approval of all labeling materials in line with Vietnam’s 2025 regulatory updates.
8. Trading License for Medical Device Distribution in Vietnam
To legally distribute Class B, C, and D medical devices in Vietnam, companies must obtain a Certificate of Eligibility for Trading Medical Devices (commonly known as a trading license for medical devices in Vietnam). This essential license confirms that the distributor meets all safety and quality requirements to handle higher-risk medical products.
Key conditions include appointing a qualified technical manager with relevant medical or engineering credentials, maintaining compliant storage facilities, and establishing documented procedures for quality monitoring, complaint handling, and product recalls.
| Document | Details |
|---|---|
| Declaration of eligibility | Official application form requesting the trading certificate. |
| Staff declaration form | Confirms employment status and assigned roles of staff. |
| House lease or loan agreement | Proof of the registered business address. |
| Vehicle registration certificate | Proof of delivery capability. |
| Technical staff qualifications | Degrees or certificates of the technical manager and key personnel. |
9. Benefits of Using a Professional Medical Device Registration Service in Vietnam
Professional services help expedite approval, ensure full compliance with MOH regulations and ASEAN Medical Device Directive Vietnam, minimize risk of delays, and streamline import and market entry.
Partnering with a professional registration service helps you:
-
Speed up approval times with expert dossier preparation
-
Ensure full compliance with Vietnam’s MOH regulations
-
Minimize risk of rejection, delays, or penalties
-
Streamline importation and market entry procedures
This is the most efficient way for foreign manufacturers to successfully register and distribute medical devices in Vietnam.
Conclusion
Successfully completing medical device registration in Vietnam is the key to unlocking growth in this dynamic and lucrative market. By following the correct classification, preparing a compliant dossier, appointing a local representative, and meeting labeling standards, you ensure fast approval and long-term market success. Ready to bring your medical devices to Vietnam? Contact Green NRJ today for expert support and a smooth, compliant market entry — seize your competitive edge now!
Related Articles:




