- If you have any questions, please contact us!
- +84 965 624 065
- info@greennrj.com.vn
Medical Device Registration in Vietnam (2025): Essential Guide to Compliance & Approval

Trusted Importer of Record (IOR) Services in Vietnam: Full Guide for Foreign Companies (2025)
March 15, 2025
The Ultimate 2025 Guide to Import Infant Formula Milk into Vietnam: Regulations, Registration & Compliance Made Easy
March 17, 2025Market Opportunities & Regulatory Overview for Medical Device Registration in Vietnam
Medical Device Registration in Vietnam is a mandatory process for manufacturers, distributors, and foreign investors aiming to enter Vietnam’s fast-growing healthcare market in 2025. Driven by rising healthcare demand, increasing public and private investment, and a growing population, Vietnam is becoming a key destination for global medical device companies.
To ensure the safety, quality, and performance of imported and domestically produced devices, the Vietnam Ministry of Health, through the Department of Medical Equipment and Construction (DMEC), enforces a robust regulatory framework. This system aligns with international standards and the ASEAN Medical Device Directive, promoting regional harmonization and regulatory transparency.
Understanding the medical device registration in Vietnam process—including medical device classification Vietnam, medical device import Vietnam, and medical device licensing Vietnam—is crucial for manufacturers and importers seeking successful market entry. This step-by-step guide explains how to register medical devices in Vietnam efficiently and comply with the latest 2025 regulatory updates. By mastering each stage of the approval cycle, you can reduce delays, meet labeling and documentation standards, and secure your position in Vietnam’s fast-evolving healthcare sector.
Legal Framework for Medical Device Registration in Vietnam
The Vietnam Ministry of Health medical device regulatory framework is founded on Decree No. 98/2021/ND-CP, effective from January 1, 2022, which regulates device management, registration, import, distribution, and usage. This decree was amended by Decree No. 07/2023/ND-CP , which clarifies procedural requirements and enhances compliance standards. Detailed guidelines on medical device classification and registration dossier requirements are outlined in Circular 05/2022/TT-BYT issued by the Ministry of Health. Additionally, regulations concerning labeling and packaging compliance are provided under Circular 18/2022/TT-BKHCN. Together, these documents form the legal basis ensuring safety, quality, and compliance of medical devices circulating in the Vietnamese market.
The MOH, via its specialized body—DMEC—is the key authority overseeing medical device registration process Vietnam, product evaluation, approval, and post-market surveillance. DMEC ensures all medical devices meet Vietnam’s safety, quality, and performance standards before they are permitted to circulate in the Vietnamese market.
According to current regulations, all medical devices require mandatory classification and registration prior to import or distribution in Vietnam. The medical device registration requirements Vietnam vary according to device risk classification, ranging from low-risk Class A devices to high-risk Class D devices. Compliance with these laws is crucial to avoid regulatory delays, fines, or product recalls.
For foreign manufacturers and distributors, understanding and following the latest MOH decrees and guidelines ensures smooth market entry and continued business operations within Vietnam’s growing medical device sector.
Vietnam Medical Device Classification: Risk Classes and Importance
The Vietnam medical device classification system plays a vital role in the medical device registration process Vietnam. Based on Circular 05/2022/TT-BYT and aligned with the ASEAN Medical Device Directive (AMDD), the system categorizes devices by intended use and risk level—ranging from Class A to Class D. Accurate medical device classification Vietnam is essential, as it directly determines regulatory pathways, required documentation, and timelines for Vietnam medical device approval.
Devices are classified into four risk-based categories:
-
Class A (Low Risk)
General medical devices with minimal risk to users or patients.
Examples: Medical bandages, surgical gloves, cotton swabs, stethoscopes. -
Class B (Low to Moderate Risk)
Devices that present slightly more risk than Class A and may require more oversight.
Examples: Hypodermic syringes, diagnostic ultrasound equipment, infusion sets. -
Class C (Moderate to High Risk)
Devices that support or sustain life, pose moderate risk, or are used in critical situations.
Examples: Infusion pumps, anesthesia machines, blood dialysis equipment, ventilators. -
Class D (High Risk)
Implantable or life-sustaining devices with the highest level of risk.
Examples: Pacemakers, implantable defibrillators, heart valves, orthopedic implants.
Important Classification Guidelines:
For combination or multifunctional devices, classification is based on the component with the highest risk. Misclassification can lead to delays or rejection during the step-by-step medical device registration Vietnam process. Consulting experienced regulatory professionals or a local authorized representative helps ensure compliance and streamline your device’s market entry.
Proper classification not only facilitates approval but also ensures that your device meets all requirements under Vietnam’s updated regulatory framework for 2025.
Essential Procedures and Compliance for Medical Device Registration in Vietnam
Vietnam enforces clear medical device registration requirements Vietnam, including detailed procedural and documentation obligations that manufacturers and distributors must comply with for timely approval.
With medical devices classified as Class A and B, the registration procedure primarily involves declaration of applicable standards via the DMEC online portal. However, some devices may require additional technical dossiers or supplementary certifications. All registration documents must be translated into Vietnamese and notarized by competent authorities. Processing times may be extended if additional information or in-depth evaluations are necessary.
For Class C and D devices, registration is mandatory if the device is listed in the licensing-required category under Circular 05/2022/TT-BYT. This circular also specifies exemptions where devices are not required to obtain marketing authorization but still must be classified and comply with technical standards.
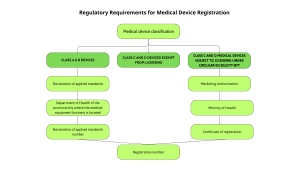
Class A Medical Devices (Low Risk)
Registration Method:
For Class A medical devices (low-risk), manufacturers or importers submit a Declaration of Applicable Standards via the DMEC online portal. The registration is then handled by the Provincial or City Department of Health where the business is established. This streamlined procedure allows faster market access by focusing on compliance with declared standards rather than full technical evaluation.
This streamlined process is specifically designed for low-risk medical devices (Class A), helping businesses quickly access the Vietnamese market without going through complex licensing procedures. Ensuring accuracy in the Declaration of Applied Standards is essential, as any errors or missing information may delay the acceptance of the device for circulation.
Approval Timeframe:
Typically, registration approval is immediate or granted within a few business days after submission, assuming all required documents are complete and accurate.
Essential Documentation Includes:
| Document | Description |
|---|---|
| Medical Device Classification Result |
Either self-declared or issued by an authorized entity, confirming the device’s low-risk status. |
| Free Sale Certificate (FSC) | Provided by the competent authority in the country of origin, certifying that the device is legally marketed and distributed there. |
| Owner Authorization Letter | A formal letter from the manufacturer designating a local Vietnamese representative to handle registration and ensure compliance with local regulations. |
| Vietnamese-Translated Label Samples and User Manuals | Required to ensure users in Vietnam can understand and safely operate the device. Must comply with local labeling regulations. |
| Product Technical File | A comprehensive file detailing the device’s specifications, materials, design, manufacturing processes, and basic safety and performance information. |
Class B Medical Devices (Low to Moderate Risk)
Registration Method:
Similar to Class A, Class B devices follow an online declaration procedure via the DMEC Portal but with additional oversight.
Approval Timeframe:
Registration decisions are generally made immediately or within several business days, contingent on the completeness of the submission.
Enhanced Documentation Requirements:
All documents required for Class A devices, plus:
-
Classification Result from a Qualified Independent Organization: Unlike Class A, self-declaration is not accepted; classification must be verified by an accredited body recognized by Vietnamese authorities to ensure accurate risk assessment.
Class C and Class D Medical Devices (Moderate to High and High Risk)
Registration Method:
For Class C and D devices, registration depends on inclusion in the list of devices requiring marketing authorization Vietnam (MA) as detailed in Circular 05/2022/TT-BYT. Devices on this list must submit a full registration dossier to DMEC and receive an MA certificate before legal marketing. Devices exempt from this list do not require MA but must still undergo classification and meet applicable standards.
Devices requiring a Marketing Authorization
If the medical device is included in the list of devices that require a license, the following procedure must be followed:
-
Submit a complete Marketing Authorization application to the Department of Medical Equipment and Construction (DMEC), under the Ministry of Health (MOH).
-
The application is reviewed by the MOH’s Appraisal Council.
-
Upon approval, the applicant will receive a Certificate of Registration along with a Registration Number (số lưu hành).
-
Only after obtaining this Certificate and Registration Number can the Class C or D medical device be legally circulated in Vietnam.
This process is mandatory for all moderate to high-risk medical devices that fall under the controlled list in Vietnam.
Devices exempt from Marketing Authorization
If the Class C or D medical device is not listed in the licensing-required category (i.e., it is included in the exemption list under Circular 05/2022/TT-BYT), then:
-
No Marketing Authorization or Certificate of Registration is required.
-
Instead, the business only needs to conduct the Medical Device Classification process to determine the appropriate risk class.
-
Once the classification is approved, the device can be imported, distributed, and used without undergoing full registration.
This exemption significantly reduces the time and administrative burden for businesses dealing with low-volume or lower-risk Class C and D devices.
Approval Timeframe for Marketing Authorization
The typical timeframe for evaluation and issuance of a Marketing Authorization (MA) and Certificate of Registration for Class C and D devices is 30 to 60 working days from the date of formal application acceptance by DMEC. Processing duration may vary based on dossier completeness, DMEC workload, and device complexity.
However, this timeframe for registration approval may vary depending on several factors, including:
-
The completeness and accuracy of the submitted dossier
-
The current workload and processing capacity of the DMEC
-
The need for additional documents or clarifications during the appraisal process
-
The complexity or novelty of the medical device
Applicants are advised to ensure all documents meet the required standards to avoid delays and to monitor the process closely via the DMEC online portal.
Comprehensive Documentation Checklist for Marketing Authorization
The registration dossier must include detailed technical documentation as required by Circular 05/2022/TT-BYT, including quality control reports, design documentation, maintenance instructions, and related certificates. All foreign-language documents must be translated and notarized into Vietnamese. For implantable or high-risk devices, clinical data from trials or equivalent evidence of safe use in international markets are necessary.
| Document | Description |
|---|---|
| Official Classification Result | Issued by an accredited regulatory body verifying the medical device’s risk class (A, B, C, or D). |
| Free Sale Certificate (FSC) | Confirms lawful distribution of the product in its country of origin or other approved foreign markets. |
| ISO 13485 Certification | Proof of a certified Quality Management System (QMS) compliant with international manufacturing standards. |
| Clinical Evaluation Report | Provides clinical data or an equivalent dossier demonstrating the device’s safety and effectiveness. |
| Risk Management File | Detailed analysis of identified risks and corresponding mitigation measures, in accordance with ISO 14971 standards. |
| Quality Control Standards Documentation | Documents the manufacturer’s internal quality standards to ensure consistent production and product performance. |
| Owner Authorization Letter | Official letter from the foreign manufacturer authorizing a Vietnamese company to act as its local representative for registration and compliance. |
| Vietnamese-Language Labels and IFU | Label samples and Instructions for Use (IFU) translated into Vietnamese, compliant with Vietnam’s medical device labeling regulations. |
Additional Considerations:
-
For Class D devices, particularly those that are implantable or life-sustaining (e.g., pacemakers, heart valves), clinical trial data may be required if the device lacks prior regulatory approval or significant use in developed countries. This ensures the highest patient safety standards and regulatory compliance.
5. Step-by-Step Medical Device Registration Process
Successfully registering medical devices in Vietnam requires following a clear, regulated process designed to ensure product safety and regulatory compliance. Below is a detailed step-by-step procedure for foreign manufacturers and distributors aiming to enter the Vietnamese medical device market in 2025.
Step 1: Medical Device Classification by Licensed Vietnamese Organization
-
Engage a Ministry of Health (MOH) licensed classification organization in Vietnam to conduct the official classification of your medical device.
-
Obtain the classification result certificate, which determines the device’s regulatory pathway and documentation requirements.
-
This is the mandatory first step for all medical devices before registration submission.
Step 2: Appointment of a Local Authorized Representative (Legal Entity)
-
Foreign manufacturers are required to appoint a Vietnam-based Marketing Authorization Holder (MAH), which can be a local legal entity or an authorized agent, to handle all regulatory communications and compliance for product registration in Vietnam.
-
The authorized representative holds the Marketing Authorization (MA) number once issued.
-
This entity will handle dossier submission, respond to authority queries, and ensure ongoing regulatory compliance.
Step 3: Dossier Preparation According to Device Class
-
Prepare a comprehensive registration dossier tailored to the device class (A, B, C, or D) as per Vietnamese regulations.
-
Ensure that all foreign-language documents are translated into Vietnamese by certified translators.
-
Notarize or legalize key documents as required to meet Vietnam’s regulatory standards.
-
Typical dossier components include: classification certificate, Free Sale Certificate (FSC), quality system certificates (e.g., ISO 13485), clinical evaluation reports, risk management files, labels and instructions in Vietnamese, and owner authorization letter.
Step 4: Application Submission via DMEC Online Portal
-
For Class A and B devices:
Submit a Declaration of Applicable Standards through the official DMEC online portal. This streamlined process facilitates faster approval for low and moderate-risk devices. -
For Class C and D devices:
Submit the full registration dossier electronically via the DMEC portal. The Ministry of Health will conduct a detailed technical evaluation, which may include requests for supplementary information or clarifications.
Step 5: Evaluation, Approval, and Issuance of Marketing Authorization Number
-
Class A and B devices:
Once the application is complete and compliant, the system usually grants instant or near-instant approval, generating the registration number immediately. -
Class C and D devices:
The MOH will perform a comprehensive dossier review, taking approximately 30 to 60 working days. Upon successful evaluation, the Ministry will issue the Marketing Authorization (MA) number (số lưu hành), allowing legal importation and distribution within Vietnam.
Following this medical device registration process in Vietnam ensures compliance with the Vietnam Ministry of Health medical device regulations, timely approval, and legal market access.
Why Following This Registration Procedure is Vital for Market Access in Vietnam
Adhering strictly to this step-by-step registration process ensures:
-
Compliance with Vietnam’s updated 2025 medical device regulations
-
Faster approval timelines by minimizing procedural errors
-
Avoidance of costly delays or application rejections
-
Legal market access and enhanced credibility with healthcare providers and regulators
For foreign medical device companies seeking growth in Vietnam’s rapidly expanding healthcare market, understanding and executing this registration workflow with precision is essential for sustainable business success.
6. Role of the Marketing Authorization Holder (MAH)
Foreign medical device manufacturers must appoint a local entity in Vietnam to act as the Marketing Authorization Holder (MAH). The MAH is the official legal representative responsible for all regulatory matters related to the device registration and market compliance.
The MAH is a legal entity registered in Vietnam and bears full responsibility for the registered medical device. This includes compliance with relevant laws and regulations, monitoring product quality after market entry, reporting adverse events, and cooperating with regulatory authorities in case of product recalls.
Key Responsibilities of the MAH:
-
Regulatory Submissions: Submit and manage registration dossiers, renewals, and updates with the Ministry of Health (MOH) and Department of Medical Equipment and Construction (DMEC).
-
Legal Compliance: Ensure all registered devices comply with Vietnamese laws, standards, and safety requirements throughout their lifecycle.
-
Post-Market Surveillance: Monitor device performance after market entry, report adverse events, and handle recalls if necessary.
-
Communication: Act as the main liaison between foreign manufacturers, Vietnamese regulators, distributors, and healthcare providers.
-
Label and Document Management: Ensure Vietnamese labels, instructions, and promotional materials meet local regulatory standards.
Appointing a reliable MAH is crucial for foreign companies to ensure smooth registration, maintain compliance, and access the growing Vietnamese medical device market effectively.
7. Vietnam Medical Devices Labeling Regulation
Mandatory Label Information for Medical Devices
According to Vietnamese regulations including Decree 43/2017/ND-CP, Decree 111/2021/ND-CP, and Circular 18/2022/TT-BKHCN, all medical device labels distributed in Vietnam must clearly display the following information in Vietnamese language:
| Label Element | Description |
|---|---|
| Medical Device Name | Official product name registered with the Ministry of Health (MOH). |
| Manufacturer/Responsible Party | Full name and physical address of the manufacturer, importer, or responsible entity. |
| Country of Origin | Country where the device was manufactured or the final processing location. |
| Registration or Import License Number | Valid Marketing Authorization (MA) or import license number issued by MOH. |
| Batch Number or Serial Number | Unique identifier for batch or device serial number to enable traceability and recall. |
| Manufacture Date and Expiry Date |
|
| Instructions for Use and Storage | Clear usage instructions and recommended storage conditions to ensure safety and product quality. |
| Warnings and Precautions | Safety warnings, contraindications, or precautionary notes if applicable. |
| Language Requirement | All labeling must be in Vietnamese. Foreign languages must be accompanied by a full Vietnamese translation. |
Consequences of Non-Compliance with Labeling Regulations
According to Decree 43/2017/ND-CP and subsequent amendments such as Decree 111/2021/ND-CP, medical device labeling must fully comply with mandatory Vietnamese-language information requirements. This includes the device name, manufacturer location, registration number, manufacturing and expiry dates, instructions for use, and safety warnings. Additionally, all accompanying promotional materials must adhere strictly to content and language regulations.
Failure to meet Vietnam’s stringent labeling requirements can lead to:
-
Regulatory penalties and fines
-
Delays or suspension of device registration approval
-
Forced product recalls or market withdrawals
-
Damage to manufacturer reputation and loss of market access
Why Proper Labeling is Critical for Medical Device Success in Vietnam
Compliant labeling not only ensures legal market access but also builds user trust and supports effective post-market surveillance and safety monitoring. Manufacturers and distributors should prioritize thorough review and approval of all labeling materials in line with Vietnam’s 2025 regulatory updates.
8. Medical Device Import Regulations Vietnam
Only Registered Medical Devices are Permitted for Import
Vietnam strictly enforces that only medical devices registered with the Ministry of Health (MOH) and possessing valid Marketing Authorization (MA) numbers can be legally imported and distributed within the country. This policy ensures that all devices on the Vietnamese market meet national safety, quality, and performance standards.
Essential Importer Requirements for Medical Device Importation:
-
Establishment License for Medical Device Distribution:
Any company or entity engaged in the importation, wholesale, or distribution of medical devices in Vietnam must hold a valid Establishment License issued by the MOH. This license certifies that the company meets the necessary conditions for safe and compliant medical device business operations, including infrastructure, qualified personnel, and quality management systems. -
Marketing Authorization Code (MAC) for Class C and D Devices:
Importers of Class C (moderate to high risk) and Class D (high risk) medical devices must obtain a valid Marketing Authorization Code (MAC) as part of the regulatory approval process. The MAC is issued after successful registration and is mandatory for customs clearance and import approval, ensuring that high-risk devices meet Vietnam’s strict regulatory standards. -
Import License Requirement for Certain Devices:
Certain Class B, C, and D devices, particularly those with advanced technologies or high risk profiles, may require a separate Import License issued by MOH or authorized agencies prior to importation, in addition to Marketing Authorization. This Import License verifies compliance with regulatory and safety standards specific to import control. This license is issued by the MOH or authorized agencies following evaluation of the product dossier and regulatory compliance. The Import License verifies that the imported devices are authorized and safe for use in Vietnam.
9. Validity of Registration and Importation
Marketing Authorization (MA) License Validity
-
Once issued by the Ministry of Health (MOH), the Marketing Authorization (MA) license for medical devices in Vietnam is valid indefinitely. This means that registered medical devices can remain legally marketed and distributed in Vietnam without the need for periodic renewal, provided that all post-market compliance obligations are continuously met.
-
Manufacturers and Marketing Authorization Holders (MAHs) must still comply with any changes in regulatory requirements and ensure timely reporting of safety information and product modifications.
- Import licenses issued prior to December 31, 2021, for Class C and D devices remain valid until December 31, 2024. After this date, importers must obtain new import permits or ensure their devices are fully registered under the current regulatory framework to continue legal importation and distribution in Vietnam.
Import License Validity for Class C and D Medical Devices
-
According to Decree No. 07/2023/ND-CP, import licenses for Class C and Class D medical devices that were issued during the period 2018 to 2021 remain valid only until December 31, 2024.
-
After this date, importers must obtain new import licenses or ensure that their devices are fully registered under the current regulatory framework to continue legal importation and distribution.
-
This transitional provision aims to harmonize older licenses with Vietnam’s updated medical device regulatory system.
10. Benefits of Using a Professional Medical Device Registration Service in Vietnam
Professional services help expedite approval, ensure full compliance with MOH regulations and ASEAN Medical Device Directive Vietnam, minimize risk of delays, and streamline import and market entry.
Partnering with a professional registration service helps you:
-
Speed up approval times with expert dossier preparation
-
Ensure full compliance with Vietnam’s MOH regulations
-
Minimize risk of rejection, delays, or penalties
-
Streamline importation and market entry procedures
This is the most efficient way for foreign manufacturers to successfully register and distribute medical devices in Vietnam.
Conclusion
Understanding and following the medical device registration in Vietnam, including the medical device registration process Vietnam, Vietnam medical device classification, and medical device import regulations Vietnam is essential for successful market entry. Partnering with experts familiar with the Vietnam Ministry of Health medical device regulatory framework and marketing authorization Vietnam (MA certificate) procedures guarantees smoother approval and sustainable growth.
For professional assistance, Green NRJ is ready to guide you every step of the way and ensure a smooth market entry process.
📢 Let’s get your products registered in Vietnam today! Contact us for a free consultation.
Related Articles:


